Cyril Roger Brossard
Well-known member
- Joined
- Aug 30, 2012
- Messages
- 408
In addition to what was discussed in this thread: Environmentally (and socially) responsible pearl farming? .
As seen in post#31
Allow me to add the two articles on the subject.
As mentioned before acidification is more a problem of dissolution than absence or reduction of nacre (shell) creation, hence "it melts faster than it deposits."
Article 1.
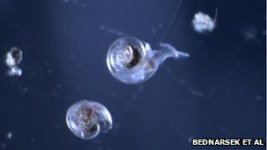
The shells of some sea snails in the Southern Ocean are already dissolving as a result of ocean acidification, according to a new study. In an analysis of free-swimming pteperods collected from Antarctic waters in 2008, scientists found that the outer layers of the animals’ shells showed signs of unusual corrosion, potential evidence that ocean acidification caused by excess carbon dioxide in the atmosphere may already be disturbing vulnerable marine species. Laboratory tests have shown that acidic water threatens many invertebrate marine species, such as clams and corals, since it hinders their ability to grow shells and exoskeletons. The most vulnerable species are those, like pteropods, that build their shells from aragonite, a form of calcium carbonate that is sensitive to increased acidity. “The corrosive properties of the water caused shells of live animals to be severely dissolved and this demonstrates how vulnerable pteropods are,” Nina Bednaršek, a scientist at the U.S. National Oceanic and Atmospheric Administration (NOAA) and lead author of the study, published in the journal Nature Geoscience, told Reuters. According to scientists, pH levels in the oceans are dropping faster than during any period over the last 300 million years.
read also this.
An Ominous Warning on the
Effects of Ocean Acidification
A new study says the seas are acidifying ten times faster today than 55 million years ago when a mass extinction of marine species occurred. And, the study concludes, current changes in ocean chemistry due to the burning of fossil fuels may portend a new wave of die-offs.
by carl zimmer
The JOIDES Resolution looks like a bizarre hybrid of an oil rig and a cargo ship. It is, in fact, a research vessel that ocean scientists use to dig up sediment from the sea floor. In 2003, on a voyage to the southeastern Atlantic, scientists aboard the JOIDES Resolution brought up a particularly striking haul.
They had drilled down into sediment that had formed on the sea floor over the course of millions of years. The oldest sediment in the drill was white. It had been formed by the calcium carbonate shells of single-celled organisms — the same kind of material that makes up the White Cliffs of Dover. But when the scientists examined the sediment that had formed 55 million years ago, the color changed in a geological blink of an eye.
“In the middle of this white sediment, there’s this big plug of red clay,” says Andy Ridgwell, an earth scientist at the University of Bristol.
In other words, the vast clouds of shelled creatures in the deep oceans had virtually disappeared. Many scientists now agree that this change was caused by a drastic drop of the ocean’s pH level. The seawater became so corrosive that it ate away at the shells, along with other species with calcium carbonate in their bodies. It took hundreds of thousands of years for the oceans to recover from this crisis, and for the sea floor to turn from red back to white.
The clay that the crew of the JOIDES Resolution dredged up may be an ominous warning of what the future has in store. By spewing carbon dioxide into the air, we are now once again making the oceans more acidic.
Today, Ridgwell and Daniela Schmidt, also of the University of Bristol, are publishing a study in the journal Natural Geoscience, comparing what happened in the oceans 55 million years ago to what the oceans are Storing CO2 in the oceans comes at a steep cost: It changes the chemistry of seawater.experiencing today. Their research supports what other researchers have long suspected: The acidification of the ocean today is bigger and faster than anything geologists can find in the fossil record over the past 65 million years. Indeed, its speed and strength — Ridgwell estimate that current ocean acidification is taking place at ten times the rate that preceded the mass extinction 55 million years ago — may spell doom for many marine species, particularly ones that live in the deep ocean.
“This is an almost unprecedented geological event,” says Ridgwell.
When we humans burn fossil fuels, we pump carbon dioxide into the atmosphere, where the gas traps heat. But much of that carbon dioxide does not stay in the air. Instead, it gets sucked into the oceans. If not for the oceans, climate scientists believe that the planet would be much warmer than it is today. Even with the oceans’ massive uptake of CO2, the past decade was still the warmest since modern record-keeping began. But storing carbon dioxide in the oceans may come at a steep cost: It changes the chemistry of seawater.
At the ocean’s surface, seawater typically has a pH of about 8 to 8.3 pH units. For comparison, the pH of pure water is 7, and stomach acid is around 2. The pH level of a liquid is determined by how many positively charged hydrogen atoms are floating around in it. The more hydrogen ions, the lower the pH. When carbon dioxide enters the ocean, it lowers the pH by reacting with water.
The carbon dioxide we have put into the atmosphere since the Industrial Revolution has lowered the ocean pH level by .1. That may seem tiny, but it’s not. The pH scale is logarithmic, meaning that there are 10 times more hydrogen ions in a pH 5 liquid than one at pH 6, and 100 times more than pH 7. As a result, a drop of just .1 pH units means that the concentration of hydrogen ions in the ocean has gone up by about 30 percent in the past two centuries.
To see how ocean acidification is going to affect life in the ocean, scientists have run laboratory experiments in which they rear organisms at different pH levels. The results have been worrying — particularly for species that build skeletons out of calcium carbonate, such as corals and amoeba-like organisms called foraminifera. The extra hydrogen in low-pH seawater reacts with calcium carbonate, turning it into other compounds that animals can’t use to build their shells.
These results are worrisome, not just for the particular species the scientists study, but for the ecosystems in which they live. Some of these vulnerable species are crucial for entire ecosystems in the ocean. Small shell-building organisms are food for invertebrates, such as mollusks and small fish, which in turn are food for larger predators. Coral reefs create an underwater rain forest, cradling a quarter of the ocean’s biodiversity.
But on their own, lab experiments lasting for a few days or weeks may not tell scientists how ocean acidification will affect the entire planet. “It’s not obvious what these mean in the real world,” says Ridgwell.
One way to get more information is to look at the history of the oceans themselves, which is what Ridgwell and Schmidt have done in their new study. At first glance, that history might suggest we have nothing to worry about. A hundred million years ago, there was over five times more carbon dioxide in the atmosphere and the ocean was .8 pH units lower. Yet there was plenty of calcium carbonate for foraminifera and other species. It was during this period, in fact, that shell-building marine organisms produced the limestone formations that would eventually become the White Cliffs of Dover.
But there’s a crucial difference between the Earth 100 million years ago and today. Back then, carbon dioxide concentrations changed very slowly over millions of years. Those slow changes triggered other slow changes in the Earth’s chemistry. For example, as the planet warmed from more carbon dioxide, the increased rainfall carried more minerals from the mountains into the ocean, where they could alter the chemistry of the sea water. Even at low pH, the ocean contains enough dissolved calcium carbonate for corals and other species to survive.
...
As seen in post#31
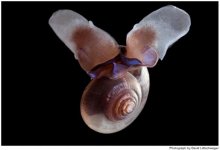
Written by Sarah Nienhuis, A. Richard Palmer and Christopher D. G. Harley -Bamfield Marine Sciences Center.As CO2 levels increase in the atmosphere, so too do they in the sea. Although direct effects of moderately elevated CO2 in sea water may be of little consequence, indirect effects may be profound. For example, lowered pH and calcium carbonate saturation states may influence both deposition and dissolution rates of mineralized skeletons in many marine organisms. The relative impact of elevated CO2 on deposition and dissolution rates are not known for many large-bodied organisms. We therefore tested the effects of increased CO2 levels—those forecast to occur in roughly 100 and 200 years—on both shell deposition rate and shell dissolution rate in a rocky intertidal snail, Nucella lamellosa. Shell weight gain per day in live snails decreased linearly with increasing CO2 levels. However, this trend was paralleled by shell weight loss per day in empty shells, suggesting that these declines in shell weight gain observed in live snails were due to increased dissolution of existing shell material, rather than reduced production of new shell material. Ocean acidification may therefore have a greater effect on shell dissolution than on shell deposition, at least in temperate marine molluscs.
Allow me to add the two articles on the subject.
As mentioned before acidification is more a problem of dissolution than absence or reduction of nacre (shell) creation, hence "it melts faster than it deposits."
Article 1.
The shells of some sea snails in the Southern Ocean are already dissolving as a result of ocean acidification, according to a new study. In an analysis of free-swimming pteperods collected from Antarctic waters in 2008, scientists found that the outer layers of the animals’ shells showed signs of unusual corrosion, potential evidence that ocean acidification caused by excess carbon dioxide in the atmosphere may already be disturbing vulnerable marine species. Laboratory tests have shown that acidic water threatens many invertebrate marine species, such as clams and corals, since it hinders their ability to grow shells and exoskeletons. The most vulnerable species are those, like pteropods, that build their shells from aragonite, a form of calcium carbonate that is sensitive to increased acidity. “The corrosive properties of the water caused shells of live animals to be severely dissolved and this demonstrates how vulnerable pteropods are,” Nina Bednaršek, a scientist at the U.S. National Oceanic and Atmospheric Administration (NOAA) and lead author of the study, published in the journal Nature Geoscience, told Reuters. According to scientists, pH levels in the oceans are dropping faster than during any period over the last 300 million years.
read also this.
An Ominous Warning on the
Effects of Ocean Acidification
A new study says the seas are acidifying ten times faster today than 55 million years ago when a mass extinction of marine species occurred. And, the study concludes, current changes in ocean chemistry due to the burning of fossil fuels may portend a new wave of die-offs.
by carl zimmer
The JOIDES Resolution looks like a bizarre hybrid of an oil rig and a cargo ship. It is, in fact, a research vessel that ocean scientists use to dig up sediment from the sea floor. In 2003, on a voyage to the southeastern Atlantic, scientists aboard the JOIDES Resolution brought up a particularly striking haul.
They had drilled down into sediment that had formed on the sea floor over the course of millions of years. The oldest sediment in the drill was white. It had been formed by the calcium carbonate shells of single-celled organisms — the same kind of material that makes up the White Cliffs of Dover. But when the scientists examined the sediment that had formed 55 million years ago, the color changed in a geological blink of an eye.
“In the middle of this white sediment, there’s this big plug of red clay,” says Andy Ridgwell, an earth scientist at the University of Bristol.
In other words, the vast clouds of shelled creatures in the deep oceans had virtually disappeared. Many scientists now agree that this change was caused by a drastic drop of the ocean’s pH level. The seawater became so corrosive that it ate away at the shells, along with other species with calcium carbonate in their bodies. It took hundreds of thousands of years for the oceans to recover from this crisis, and for the sea floor to turn from red back to white.
The clay that the crew of the JOIDES Resolution dredged up may be an ominous warning of what the future has in store. By spewing carbon dioxide into the air, we are now once again making the oceans more acidic.
Today, Ridgwell and Daniela Schmidt, also of the University of Bristol, are publishing a study in the journal Natural Geoscience, comparing what happened in the oceans 55 million years ago to what the oceans are Storing CO2 in the oceans comes at a steep cost: It changes the chemistry of seawater.experiencing today. Their research supports what other researchers have long suspected: The acidification of the ocean today is bigger and faster than anything geologists can find in the fossil record over the past 65 million years. Indeed, its speed and strength — Ridgwell estimate that current ocean acidification is taking place at ten times the rate that preceded the mass extinction 55 million years ago — may spell doom for many marine species, particularly ones that live in the deep ocean.
“This is an almost unprecedented geological event,” says Ridgwell.
When we humans burn fossil fuels, we pump carbon dioxide into the atmosphere, where the gas traps heat. But much of that carbon dioxide does not stay in the air. Instead, it gets sucked into the oceans. If not for the oceans, climate scientists believe that the planet would be much warmer than it is today. Even with the oceans’ massive uptake of CO2, the past decade was still the warmest since modern record-keeping began. But storing carbon dioxide in the oceans may come at a steep cost: It changes the chemistry of seawater.
At the ocean’s surface, seawater typically has a pH of about 8 to 8.3 pH units. For comparison, the pH of pure water is 7, and stomach acid is around 2. The pH level of a liquid is determined by how many positively charged hydrogen atoms are floating around in it. The more hydrogen ions, the lower the pH. When carbon dioxide enters the ocean, it lowers the pH by reacting with water.
The carbon dioxide we have put into the atmosphere since the Industrial Revolution has lowered the ocean pH level by .1. That may seem tiny, but it’s not. The pH scale is logarithmic, meaning that there are 10 times more hydrogen ions in a pH 5 liquid than one at pH 6, and 100 times more than pH 7. As a result, a drop of just .1 pH units means that the concentration of hydrogen ions in the ocean has gone up by about 30 percent in the past two centuries.
To see how ocean acidification is going to affect life in the ocean, scientists have run laboratory experiments in which they rear organisms at different pH levels. The results have been worrying — particularly for species that build skeletons out of calcium carbonate, such as corals and amoeba-like organisms called foraminifera. The extra hydrogen in low-pH seawater reacts with calcium carbonate, turning it into other compounds that animals can’t use to build their shells.
These results are worrisome, not just for the particular species the scientists study, but for the ecosystems in which they live. Some of these vulnerable species are crucial for entire ecosystems in the ocean. Small shell-building organisms are food for invertebrates, such as mollusks and small fish, which in turn are food for larger predators. Coral reefs create an underwater rain forest, cradling a quarter of the ocean’s biodiversity.
But on their own, lab experiments lasting for a few days or weeks may not tell scientists how ocean acidification will affect the entire planet. “It’s not obvious what these mean in the real world,” says Ridgwell.
One way to get more information is to look at the history of the oceans themselves, which is what Ridgwell and Schmidt have done in their new study. At first glance, that history might suggest we have nothing to worry about. A hundred million years ago, there was over five times more carbon dioxide in the atmosphere and the ocean was .8 pH units lower. Yet there was plenty of calcium carbonate for foraminifera and other species. It was during this period, in fact, that shell-building marine organisms produced the limestone formations that would eventually become the White Cliffs of Dover.
But there’s a crucial difference between the Earth 100 million years ago and today. Back then, carbon dioxide concentrations changed very slowly over millions of years. Those slow changes triggered other slow changes in the Earth’s chemistry. For example, as the planet warmed from more carbon dioxide, the increased rainfall carried more minerals from the mountains into the ocean, where they could alter the chemistry of the sea water. Even at low pH, the ocean contains enough dissolved calcium carbonate for corals and other species to survive.
...
Last edited: