Mostawesomecoffee
New Member
- Joined
- Jun 16, 2014
- Messages
- 44
Hi,
I have two shells of the same species of freshwater mussel. Both shells were rather old when found, with the interiors entirely covered with a chalk-like layer. I'm guessing that they were lying around the lake shore where I found them for at least a year, if not longer. I cleaned both shells with sandpaper, then with 0000 stel wool. The first picture is of the nacre of the larger shell after being cleaned in this manner. The subsequent pictures are of the smaller shell which, in addition to being subjected to the aforementioned treatments, was placed in hydrogen peroxide for a few hours. This did very little. The shell was then rinsed off, and placed in bleach (about 1:1 bleach to water ratio) for about twelve hours.
My question concerns the gold coloring seen in the nacre. I'm wondering if this is a harmless variation or a flaw. The gold coloring can be seen very clearly in the first picture (the large shell). This gold coloring looked the same in the smaller shell prior to bleaching. During bleaching, pieces of the periostracum loosened up, as well as this gold area. The overall size of the gold area shrunk. As can be seen in the picture (of the smaller shell) the gold area is rimmed with white. This white color is where the bleach has undercut the gold area. This white undercutting can be seen to a minor degree in other parts of the small shell, but not as dramatically as in the gold area. In the second photo, the gold area is/was in the deepest part of the shell.
The third picture is also of the smaller shell, but lit from the back. The gold area seems to be more opaque than the rest of the shell.
So, it seems that either the gold-colored area is a "flaw" with regard to the rest of the nacre (i.e., composed of something heavy in protein that's not as durable as the rest of the nacre) or the entire shell has been damaged by extreme bleaching, and the gold area is in no way weeker than any other part of the shell.
What are your thoughts?
Thanks.

View attachment IMG 1841
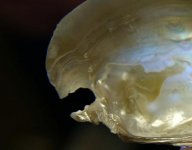
I have two shells of the same species of freshwater mussel. Both shells were rather old when found, with the interiors entirely covered with a chalk-like layer. I'm guessing that they were lying around the lake shore where I found them for at least a year, if not longer. I cleaned both shells with sandpaper, then with 0000 stel wool. The first picture is of the nacre of the larger shell after being cleaned in this manner. The subsequent pictures are of the smaller shell which, in addition to being subjected to the aforementioned treatments, was placed in hydrogen peroxide for a few hours. This did very little. The shell was then rinsed off, and placed in bleach (about 1:1 bleach to water ratio) for about twelve hours.
My question concerns the gold coloring seen in the nacre. I'm wondering if this is a harmless variation or a flaw. The gold coloring can be seen very clearly in the first picture (the large shell). This gold coloring looked the same in the smaller shell prior to bleaching. During bleaching, pieces of the periostracum loosened up, as well as this gold area. The overall size of the gold area shrunk. As can be seen in the picture (of the smaller shell) the gold area is rimmed with white. This white color is where the bleach has undercut the gold area. This white undercutting can be seen to a minor degree in other parts of the small shell, but not as dramatically as in the gold area. In the second photo, the gold area is/was in the deepest part of the shell.
The third picture is also of the smaller shell, but lit from the back. The gold area seems to be more opaque than the rest of the shell.
So, it seems that either the gold-colored area is a "flaw" with regard to the rest of the nacre (i.e., composed of something heavy in protein that's not as durable as the rest of the nacre) or the entire shell has been damaged by extreme bleaching, and the gold area is in no way weeker than any other part of the shell.
What are your thoughts?
Thanks.

View attachment IMG 1841

